
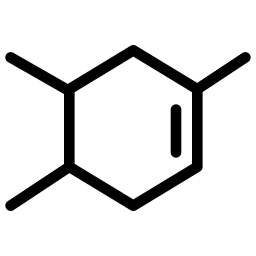
ALTU-135
Thuốc Gốc
Biotech
Cơ Chế Tác Dụng :
Chỉ Định :
Dữ Kiện Thương Mại
Tài Liệu Tham Khảo Thêm
drugbank
Bạn thấy hài lòng ?
Bạn chưa hài lòng ?
... loading
... loading